Results with RYSTIGGO

Results with RYSTIGGO
RYSTIGGO was studied in a large clinical trial (200 participants) for people with gMG
The clinical trial included adults with gMG who were anti-AChR or anti-MuSK antibody positive. These participants were divided into 3 groups:
- 66 people taking 7 mg/kg of RYSTIGGO
- 67 people taking 10 mg/kg of RYSTIGGO
- 67 people taking a placebo
During the treatment period, participants received 1 dose each week for 6 weeks, in addition to their current gMG treatment. This was followed by an observation period of up to 8 weeks.

UCB is grateful to all the participants, doctors, and nurses who took part in the RYSTIGGO clinical trial.

Significant improvement in the activities of daily living
Participants receiving RYSTIGGO experienced significant improvement in MG-ADL by the end of the 6-week treatment period.*
*As determined by improvement from baseline on Day 43 of the study; −3.4 points in the RYSTIGGO-treated group (133 people) vs −0.8 in the placebo group (67 people). Individual results may vary, and not all people taking RYSTIGGO will experience improvements.
About the MG-ADL scale used in the clinical trial
The main results in the trial used the MG-ADL (Myasthenia Gravis Activities of Daily Living) scale, a tool doctors use to measure participants' ability to complete 8 functional activities commonly affected by gMG:
- Talking
- Chewing
- Swallowing
- Breathing
- Brushing teeth or combing hair
- Rising from a chair
- Double vision
- Eyelid droop
Participants scored themselves, and their scores were reviewed by doctors in the trial.
A total MG-ADL score ranges from 0-24, with a lower score indicating less impairment and an improved ability to complete everyday tasks.
Download the MG-ADL Tool to learn more.
DOWNLOAD
Rapid improvement by the end of Week 6
RYSTIGGO was shown to significantly improve MG-ADL scores by the end of the 6-week treatment period. Improvements were seen in some participants as early as 1 week after their first dose.†
†35% (23 out of 66 people) of the 7 mg/kg group and 38% (25 out of 66) of the 10 mg/kg group responded to treatment by Day 8, compared with 24% (16 out of 67) of the placebo group.

Up to 72% responded to RYSTIGGO treatment
Among clinical trial participants receiving RYSTIGGO, most saw their symptoms improve.‡
Caution must be used when interpreting as conclusions cannot be drawn. Results may vary.
‡72% (46 out of 64 people) in the 7 mg/kg group and 69% (43 out of 62) in the 10 mg/kg group, compared with 31% (20 out of 64) in the placebo group. Response was defined as at least a 2-point improvement in MG-ADL score from baseline by Day 43.

RYSTIGGO is the first targeted treatment approved for anti-MuSK antibody-positive gMG
All 12 participants with anti-MuSK antibody-positive gMG receiving RYSTIGGO showed improvement in their MG-ADL scores.§
Caution must be used when interpreting as conclusions cannot be drawn. Results may vary.
§The study did not compare improvements in anti-MuSK antibody-positive participants to those who were anti-AChR antibody-positive and received RYSTIGGO (120 participants).

Over 25% of participants receiving RYSTIGGO in the clinical trial achieved MSE
MSE is sometimes used as a treatment goal for gMG, and is defined as a total MG-ADL score of 0 or 1. People who reach MSE experience minimal symptoms as assessed by the MG-ADL scale.
MSE was an exploratory outcome in the clinical trial, not a primary or secondary outcome. Caution must be used when interpreting as conclusions cannot be drawn. Results may vary.

26% (17 out of 66 people) in the 7 mg/kg group and 28% (19 out of 67 people) in the 10 mg/kg group, compared with 3% (2 out of 67 people) in the placebo group.

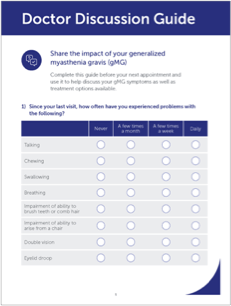
Talk to your doctor about RYSTIGGO and your gMG treatment goals
The Doctor Discussion Guide can help you get the conversation started.
The safety of RYSTIGGO in the clinical trial
This chart includes side effects experienced by at least 5% of people treated with RYSTIGGO during the clinical trial. They were also seen more often in the RYSTIGGO group than in the placebo group.
(133 people)
(67 people)
RYSTIGGO may cause serious side effects, including:
- Infection: RYSTIGGO may increase the risk of infection. Your healthcare provider should check you for infections before starting and during treatment with RYSTIGGO. Tell your healthcare provider if you have any history of infections. Tell your healthcare provider right away if you have signs or symptoms of an infection during treatment with RYSTIGGO. Some of the signs and symptoms may include fever, chills, frequent and/or painful urination, cough, runny nose, wheezing, shortness of breath, fatigue, sore throat, excess phlegm, nasal discharge, back pain, and/or chest pain.
- Aseptic Meningitis: RYSTIGGO could cause aseptic meningitis. Tell your healthcare provider right away if you develop any signs or symptoms of meningitis during treatment with RYSTIGGO such as severe headache, neck stiffness, drowsiness, fever, sensitivity to light, painful eye movements, nausea, and vomiting.
- Hypersensitivity Reactions: RYSTIGGO can cause swelling and rash. Your healthcare provider should monitor you during and after treatment and discontinue RYSTIGGO if needed. Tell your healthcare provider immediately about any undesirable reactions you experience after administration.


